In the environment there are currently several types of atoms
Each atom is different from all other atoms
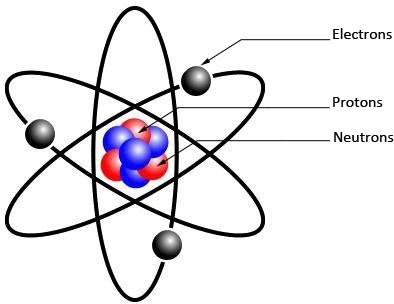
The atom is composed of two components
I / core: and that is composed of two components which are called nucleons (The number Nucleon denoted A)
It is 1836 times lower than that of the proton.
1/the protons: positively charged particles , its electric charge qp called elementary charge is equal to
qp= e = 1.6x10-19C
The proton mass mp is very low. It is equal to
mp=1,67x10-27 Kg
mp=1,67x10-27 Kg
Protons of all nuclei are identical, they are different from one atom to another by their number (Rated Z)
2/The neutrons: as its name suggests, the neutron is an uncharged particle
The mass of the neutron mn is close to that of the proton. It is equal to mn=1,67x10-27Kg
The mass of the neutron mn is close to that of the proton. It is equal to mn=1,67x10-27Kg
(The number of neutrons rated N)
II / The electrons
The electron carries an electric charge qe negative equal to the opposite of the elementary charge
Qe= -e = -1,6x10-19 C
The mass of an electron is extremely low and valleys 9,1x10-31 Kg
Electrons of all atoms are the same, they differ only by their number
In the basic state is the number of electrons equal to the number Z the number of protons contained in the core
Note: The mass of an electron is negligible
A ring containing Z proton electric charge equal to the sum of the charges of protons that compose it. Like all protons are identical and have the same elementary charge e then in charge of a nucleus containing Z protons is equal to:
Qnoyau =Zxe
The weight of a core consisting of Z protons and neutrons N is given by the relation:
M noyau = Z.mp + N mp
As the masses of the proton and neutron are almost equal, then:
M noyau=Zmp+Nmp=A.mp=Ax1,67x10-27 Kg
The electron mass is much smaller than that of the core can be considered as the mass of the atom is close to that do its core.
We therefore to matome =Ax1,67x10-27
Kg
The atomic molar mass is almost equal M=Ax1,67x10-27 N ,where N is Avogadro's number as N=6,02x1023 . As a result:
The atomic molar mass is almost equal M=Ax1,67x10-27 N ,where N is Avogadro's number as N=6,02x1023 . As a result:
M=Ax1,67x10-27x6,02x1023 Kg =Ax10-3 Kg = A.g
This is why A is called mass number
Definition of a chemical element:
Atoms and ions having the same charge number Z corresponding to the same chemical element
In a chemical reaction there is conservation of chemical elements
A chemical element characterized by its atomic number Z and a symbol
Definition of isotopes:
The isotopes of a chemical element are atoms whose nuclei have the same charge number Z and the mass number A different
Atoms and ions having the same charge number Z corresponding to the same chemical element
In a chemical reaction there is conservation of chemical elements
A chemical element characterized by its atomic number Z and a symbol
Definition of isotopes:
The isotopes of a chemical element are atoms whose nuclei have the same charge number Z and the mass number A different
0 commentaires:
Post a Comment